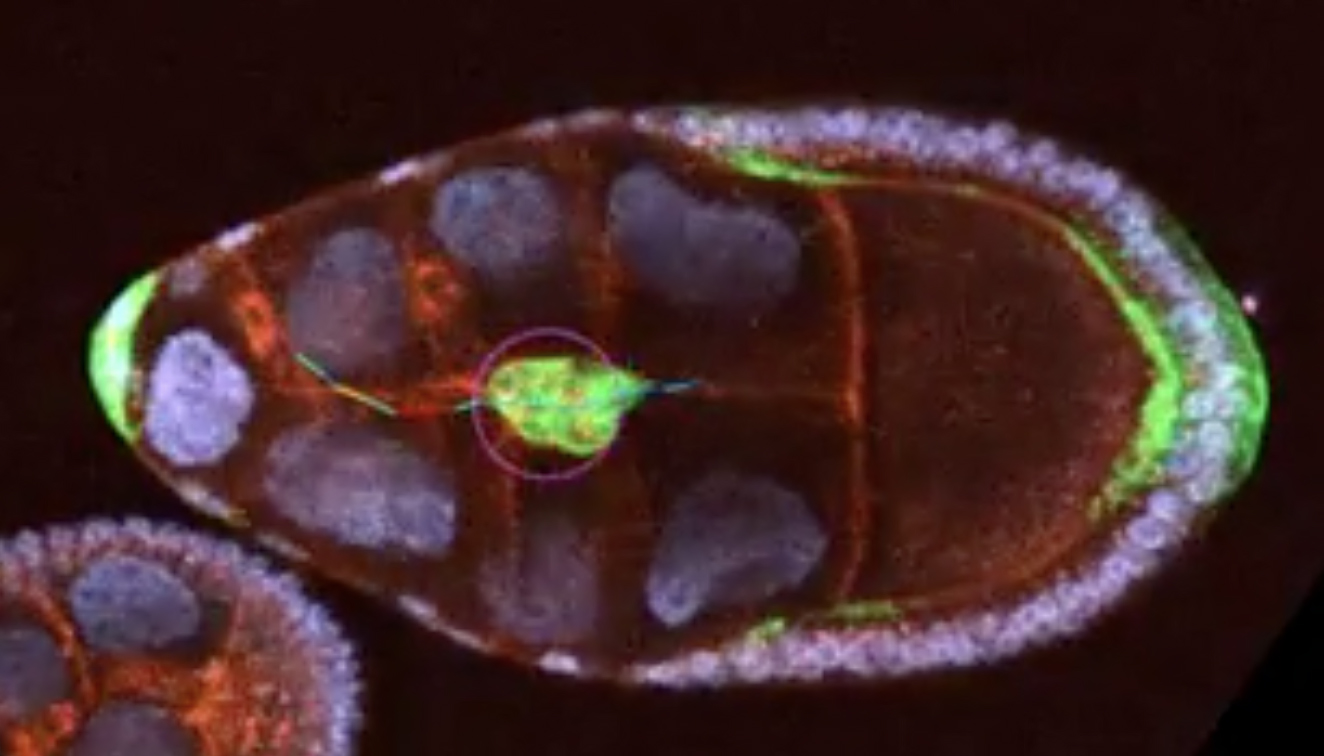
Imagine cells navigating through a complex maze, guided by chemical signals and the physical landscape of their environment. At UMBC, a team of researchers has contributed an important discovery about how cells move, or migrate, through this maze of bodily tissues. Potential implications include better understanding of diseases like cancer and advancing medical treatments.
Published in iScience, the team’s study combines biological experiments and mathematics to reveal new insights into cell migration. Alex George, Ph.D. ’24, biological sciences, and Naghmeh Akhavan, Ph.D. ’25, mathematics, led the study, which explores how cells in fruit fly egg chambers navigate their environment. Their mentors, Michelle Starz-Gaiano, professor of biological sciences, and Brad Peercy, professor of mathematics, are co-authors.
By integrating mathematical modeling with advanced imaging, the team discovered that the physical shape of the egg chamber, combined with chemical signals called chemoattractants, significantly influences how cells move.

“This paper takes an interdisciplinary focus with tight collaboration between a mathematical framework and experimental design,” Peercy says. “The results promote the idea that complex distribution of chemical attractants can explain specific variations in migratory movement.” His enthusiasm highlights the study’s innovative approach, which merges precise mathematical models with real-world biological experiments to uncover patterns that were previously invisible.
Following the breadcrumbs
The team’s work focuses on border cells, a type of cell in fruit fly egg chambers, which are a model system for studying cell migration because of their similarities to processes in human development and disease. The team found that the border cells’ movement wasn’t just driven by continuously increasing chemical concentrations from one end of the egg chamber to the other, as earlier models suggested. Instead, the physical structure of the tissue—narrow tubes alternating with wider gaps—played a critical role.
“This was the first time that we characterized that there were these patterns of migration behavior that ended up correlating to aspects of the tissue geometry,” explains George, who specializes in capturing live images of these cells. He likens the process to Hansel and Gretel following breadcrumbs through a forest: On a flat plain, the trail is clear, but in a landscape with ravines and valleys, the breadcrumbs pool in unexpected ways, complicating the path.

To understand this, Akhavan developed mathematical models that simulate how cells respond to both chemical signals and tissue geometry together. “Alex’s experiments showed that the speed is not exactly the way previous models showed it,” she says. Her models revealed that cells speed up in narrow tubes and slow down in larger gaps, a pattern confirmed by George’s imaging.
Both approaches—wet-lab experiments and modeling—bring unique strengths to the work. Putting them together “is like unveiling the invisible from two different perspectives,” George says. “My experiments would refine her model, and her model would refine my experiments.”
And then, “When our model shows exactly what Alex found in his experiments, we love that,” Akhavan adds.
Learning new languages
This synergy didn’t always come easily. Working across disciplines meant learning to speak each other’s scientific “languages.” Akhavan, with a background in pure mathematics, recalls that when she joined the project in spring 2022, “Everything was in a different language for me.” Similarly, “A couple of times I opened my MATLAB code and Alex’s eyes got huge,” Akhavan laughs.
Yet, their collaboration flourished, fostering not only scientific breakthroughs but also friendship. “It’s a challenge to communicate across disciplines since it’s almost like speaking in different languages,” Starz-Gaiano says. “Both Alex and Naghmeh got more adept at explaining their work and honing their research questions as a result of working together over a couple of years, which was great to watch.”
Putting together wet lab experiments and mathematical modeling “is like unveiling the invisible from two different perspectives. My experiments would refine her model, and her model would refine my experiments.”
Alex George, Ph.D. ’24, biological sciences
“It is a risky and vulnerable situation to be open with colleagues in areas in which you are not a burgeoning expert,” Peercy adds. “Naghmeh and Alex have grown so much through this project to genuinely rely on each other’s opinion.”
The study’s broader impact lies in its potential to inform fields beyond developmental biology. Cell migration is critical in processes like wound healing, immune responses, and cancer metastasis. “Most research on how cells navigate the world has focused only on chemical signals or only on structural ones, so this is one of the first studies to consider how those two things impact each other, which is likely to be relevant in many cases,” Starz-Gaiano explains. By showing how tissue geometry and chemical signals interact, the research could guide new strategies for controlling cell movement via medical treatments.



New strategies lead to new discoveries
George refined his expertise in microscopy through working with Tagide deCarvalho in UMBC’s Keith R. Porter Imaging Facility. “It helped me learn a lot, getting my hands on other people’s work and visualizing all the cool things,” he says. “A picture is worth a thousand words, but a movie? Ten thousand words.” Now he’s taking his skills to the Dartmouth Cancer Center’s microscopy core facility at the Geisel School of Medicine, where he’ll start as a research scientist in June.
For Akhavan and George, leading this project has been a defining experience. Akhavan’s models, including a new approach that uses energy calculations to better capture the egg chamber’s complex geometry, have become a cornerstone of her dissertation, and she plans to continue this work post-graduation.
George and Akhavan’s mentors played a pivotal role in their success. “Michelle is a role model for me,” Akhavan says, praising the collaborative spirit of Starz-Gaiano and Peercy. “Dr. Peercy and Dr. Starz-Gaiano make the best combination for doing interdisciplinary research. This collaboration is amazing.”


The team’s work continues to evolve, including recent experiments at the Advanced Imaging Center at the Janelia Research Campus in Virginia, where George used advanced microscopes to capture previously unseen dynamics of the relevant chemoattractants. These findings will further refine their models, opening new avenues for research.
“We are developing new experimental strategies both on the biology and the math side of things,” Starz-Gaiano says, “so it will be exciting to see where this will take us next.”
Tags: Biological Sciences, Biology, CNMS, GradResearch, Mathematics and Statistics, MathStat, Research